PART 1. PRINCIPLES OF ATOMIC AND MOLECULAR STRUCTURE AND CHEMICAL REACTIVITY.
Chapter 1. Atomic Structure
Chapter 2. The Octet Rule and Lewis Structures
Chapter 3. Representing Organic Structural Formulas
Chapter 4. Covalent Bonds: Energetics and Properties
Chapter 5. Bond Length and Bond Strength
Chapter 6. Electronegativity and Bond Polarity
Chapter 7. Formal Charge
Chapter 8. Molecular Shapes: Valence Shell Electron Pair Repulsion Theory
Chapter 9. Atomic Orbitals
Chapter 10. Orbital Hybridization
Chapter 11. The Functional Groups
Chapter 12. Physical Properties and Molecular Structure
Chapter 13. Lewis Acids and Bases
Chapter 14. Isomers and Stereochemistry
Chapter 15. Introduction to Organic Reactions
Chapter 16. Organic Reaction Mechanisms: A General Overview
Chapter 17. Steric Hindrance
PART 2. THE FUNCTIONAL GROUPS: PROPERTIES AND REACTIVITY
Chapter 18. The Alkane Hydrocarbons
Chapter 19. Alkyl Halides: Substitution and Elimination Reactions
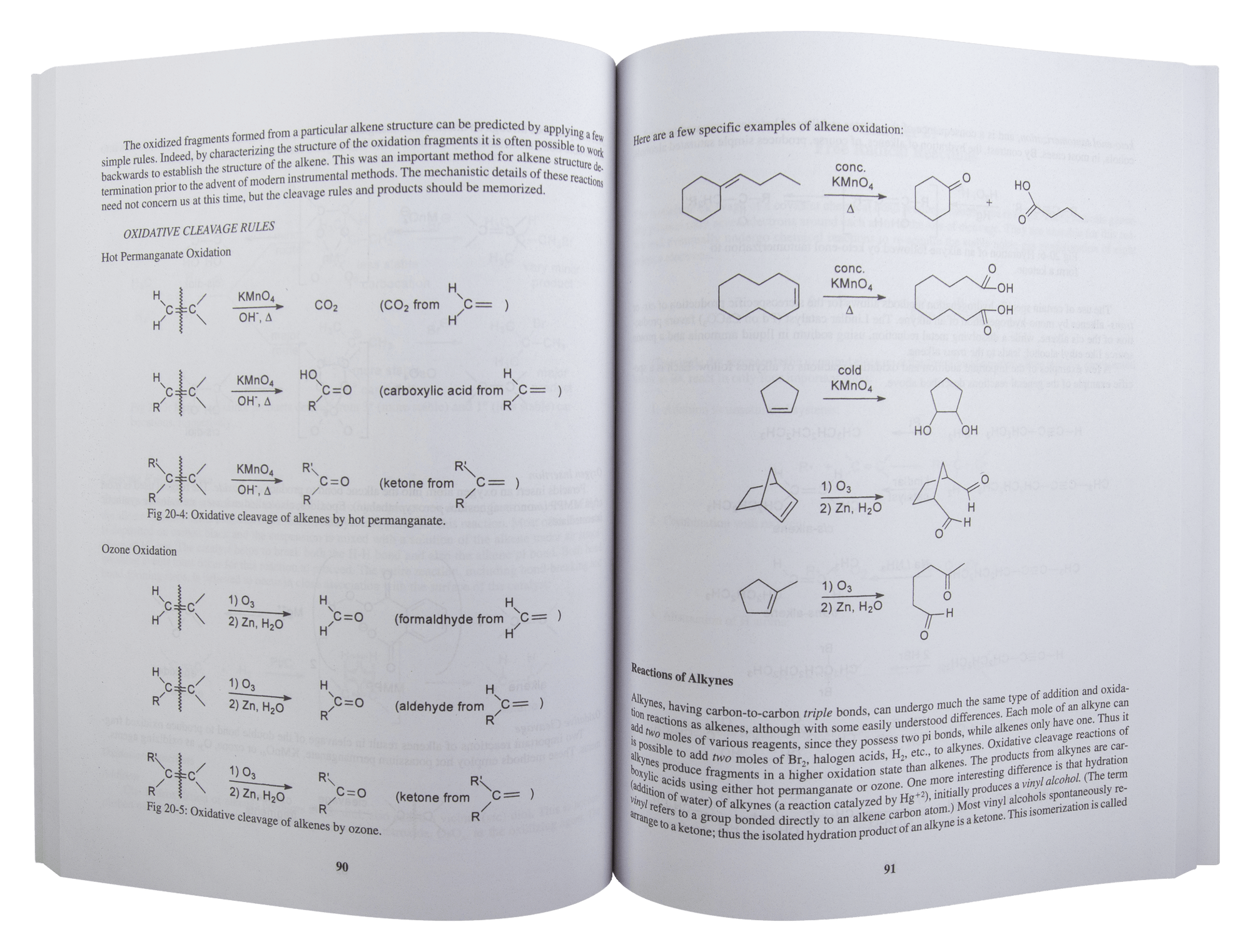
Chapter 20. The Unsaturated Hydrocarbons: Alkenes and Alkynes
Chapter 21. Free-Radical Reactions
Chapter 22. Alcohols and Ethers
Chapter 23. Addition and Substitution Reactions of Aldehydes and Ketones
Chapter 24. Oxidation and Reduction Reactions of Carbonyl Compounds
Chapter 25. Carboxylic Acids and Derivatives
Chapter 26. Reactions of the Carbonyl Group
Chapter 27. Resonance Structures and Electron Delocalization
Chapter 28. Aromatic Compounds and Aromaticity
Chapter 29. Electrophilic Aromatic Substitutions
Chapter 30. Reactions of Enolate Carbanions
Chapter 31. The Amines
Chapter 32. Overview of Reaction Mechanisms: The Production and Fate of Reactive Intermediates
PART 3. THE SPECTROSCOPIC METHODS OF ANALYSIS
Chapter 33. Introduction
Chapter 34. U-V Visible Spectroscopy
Chapter 35. Infra-Red Spectroscopy
Chapter 36. Proton NMR Spectroscopy
Chapter 37. Carbon-13 NMR
Chapter 38. Mass Spectrometry.